Pioneering AI in Signal Analytics
Reducing Signal Noise and Accelerating Discovery in Pharmaceutical Research.
-
Client
ArisGlobal
-
Headquarters
Boston, Massachusetts
-
Industry
Life Sciences
-
Product Team
1 PM | 1 DA | 12 Dev | 3 UX
-
My Role
Product Design Domain Lead
-
Time spent
2024, 4 months
Overview
In the competitive landscape of pharmaceutical research and development, our Data & Analytics domain faced a pivotal challenge: to revolutionize signal detection by reducing signal noise and becoming innovation industry leaders.
As the lead designer, I spearheaded a strategy for cutting-edge analytics and product growth, collaborating with cross-functional teams to design and implement an AI integration framework that met industry needs through user-centered design. I championed the role of design in driving user engagement and adoption, iterating strategies based on user feedback, market trends, and technological advancements.
Situation
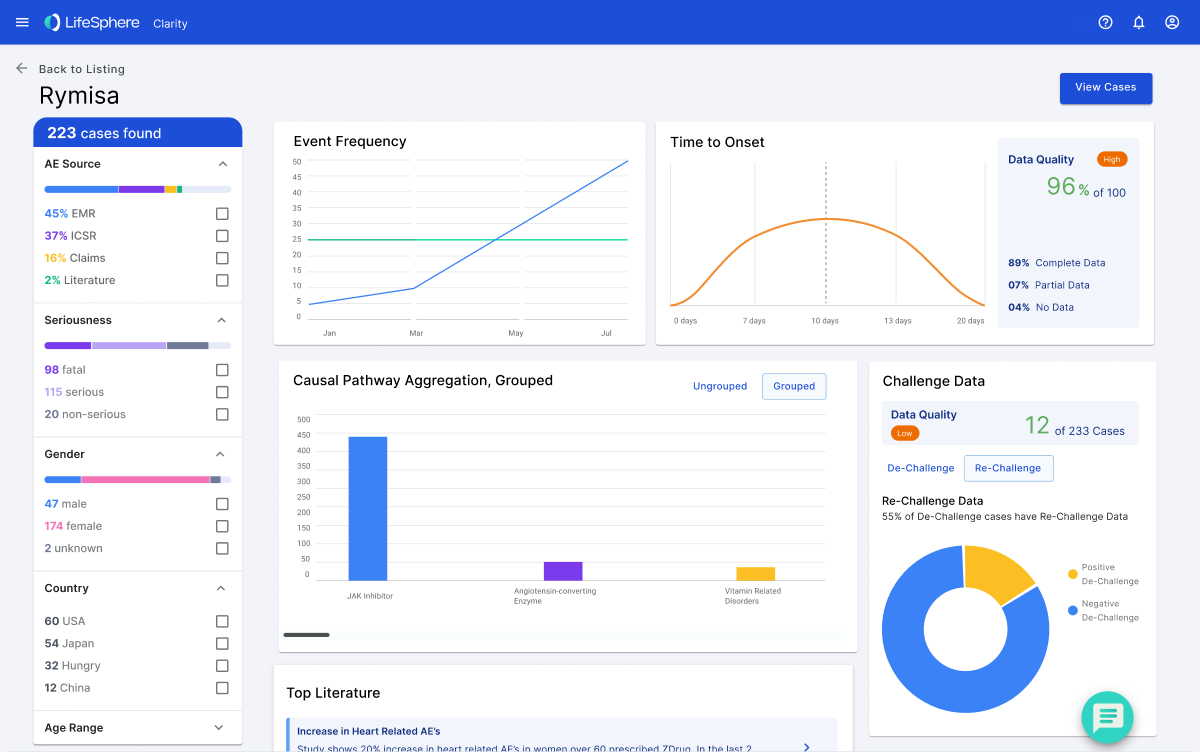
The team faced a critical bottleneck in the signal detection process, where signal noise accounted for a staggering 95% of patterns. Recognizing this challenge, we saw an opportunity to revolutionize the efficiency of drug development.
The Challenge
I faced the challenge of designing a proof of concept (POC) within a four-week timeframe. The task was to re-envision the signal detection process, aiming to minimize non-contributory signals and maximize efficiency. The team sought to empower risk and safety physicians by enabling them to focus on tasks requiring higher cognitive abilities and advanced medical reasoning. I navigated technical complexities and industry-specific requirements while establishing key partnerships for data integration.
- IC overnight 4 weeks to design POC
- Technical Complexity
- Industry Specificity
- Establishing Partnerships for data
Action
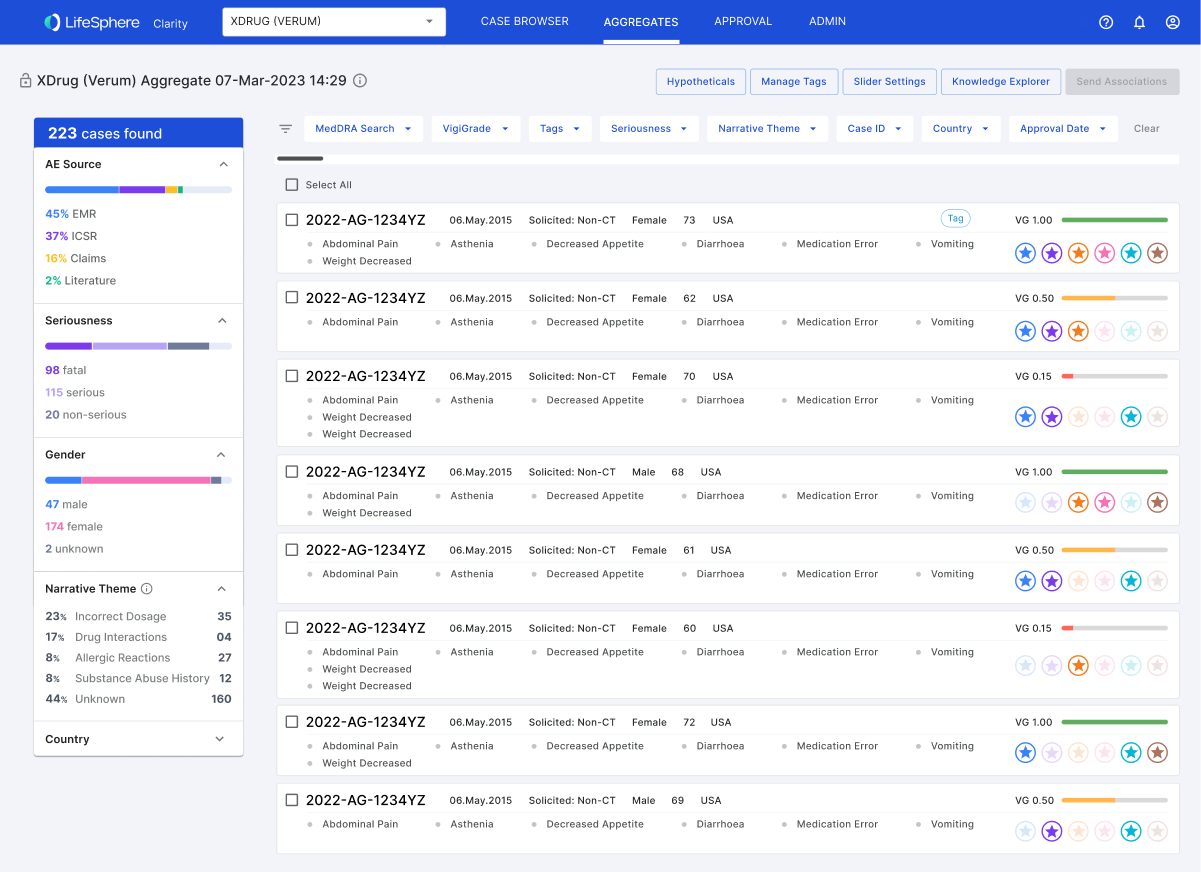
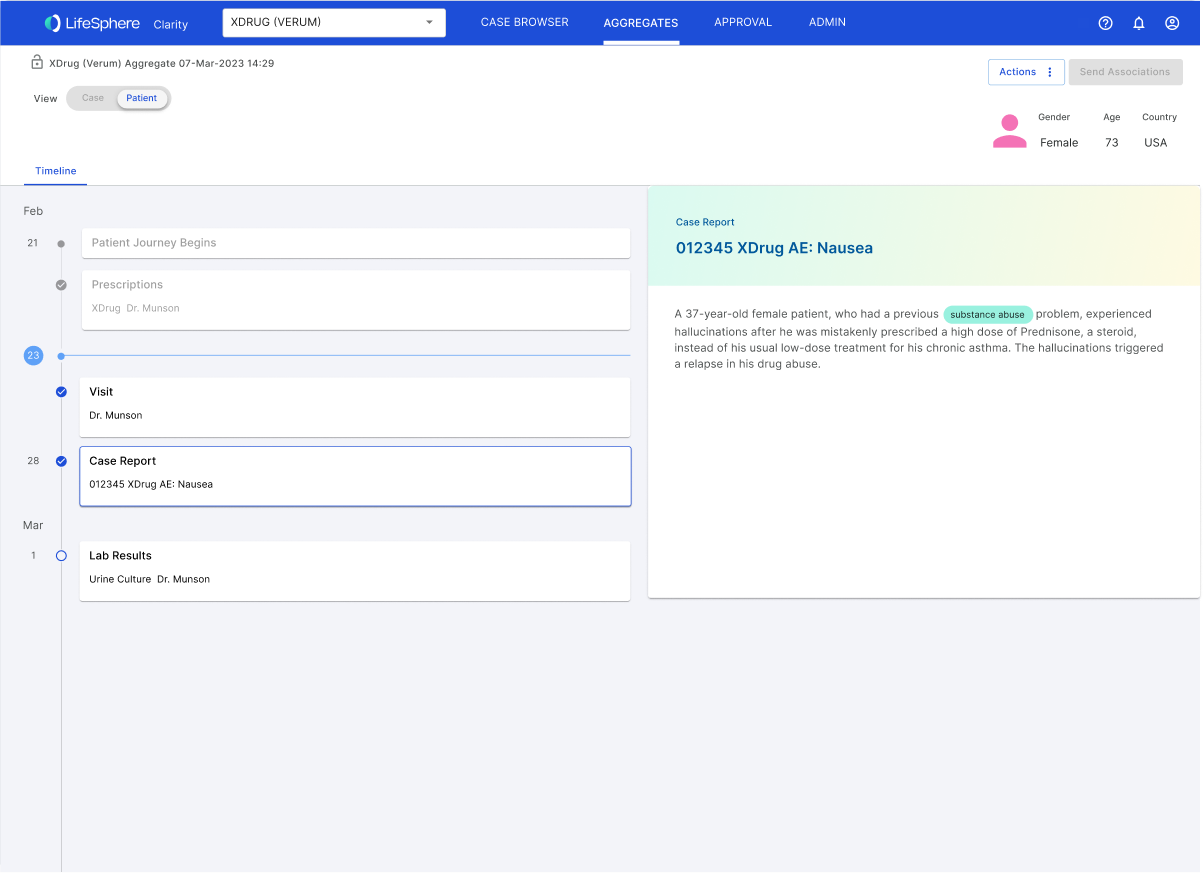
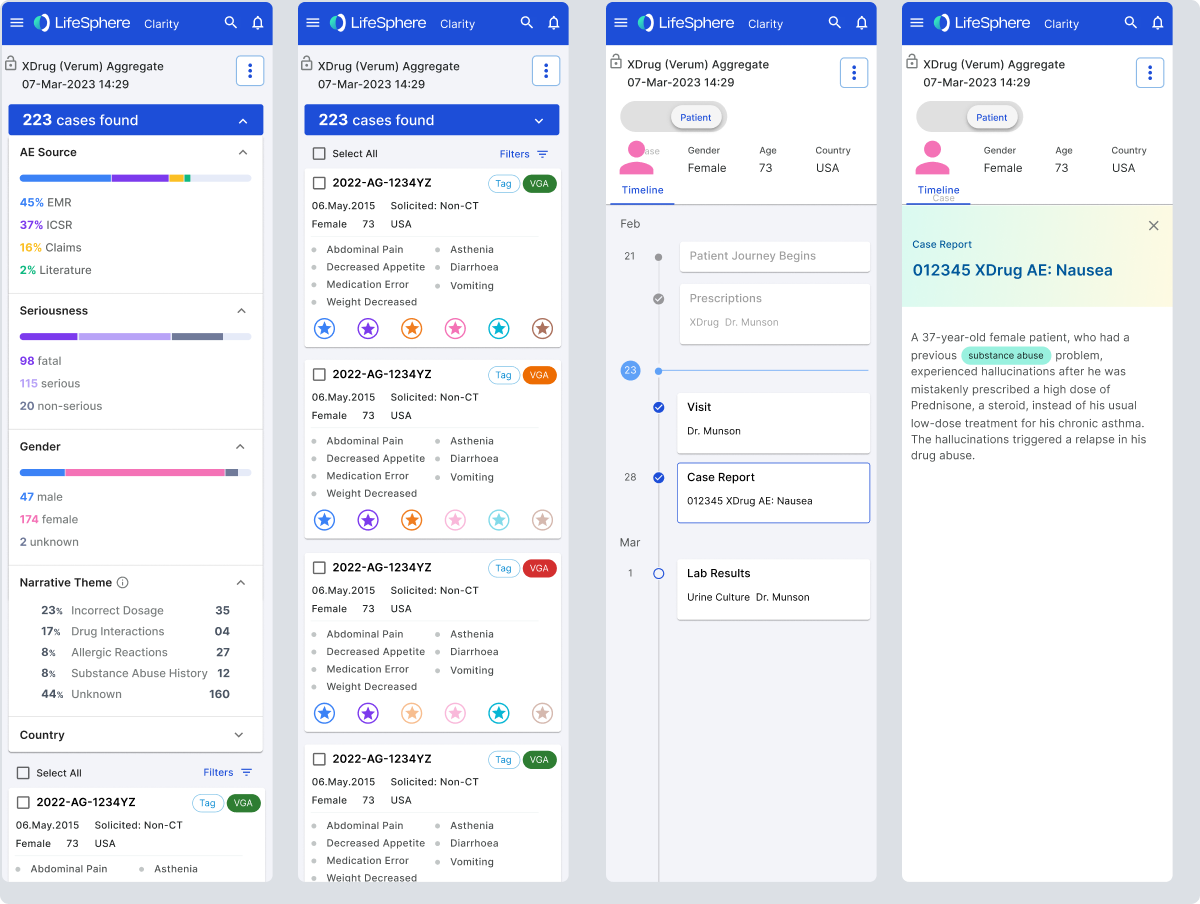
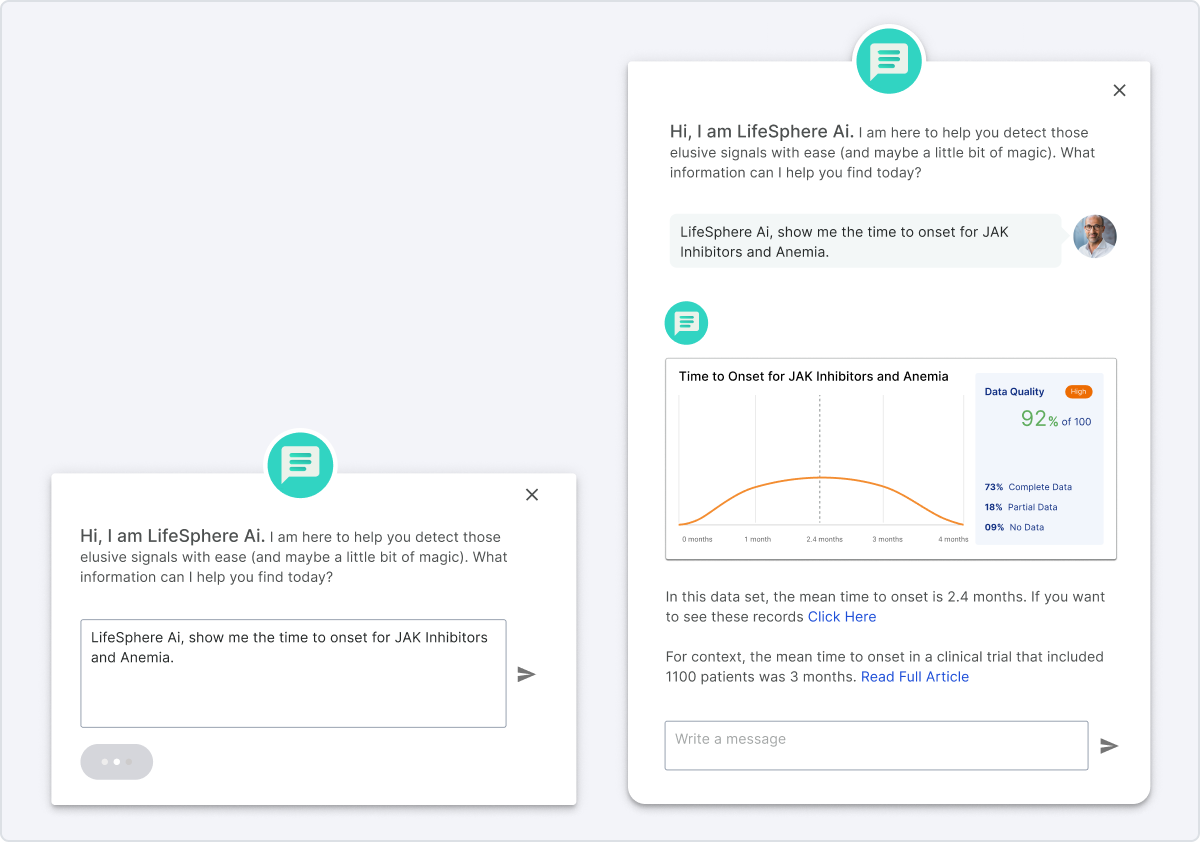
Taking a user-centric approach, the team embarked on a journey of data-driven design. We systematically analyzed known causal factors across extensive datasets, employing a methodology reminiscent of credit scoring, considering variables such as time to onset and increased frequencies. Simultaneously, we explored integrating real-world data, disrupting the traditional reliance on Individual Case Safety Reports (ICSRs) and introducing a more agile and responsive system.
Result
The outcome of these data-driven actions was a breakthrough in proactive signal detection. Our approach reduced false positives by 95% and allowed for the early identification of signals, significantly increasing accuracy and efficiency.
This translated into competitive advantage as risk and safety physicians could engage in more impactful tasks, aligning with their expertise. We secured strategic partnerships with 2 major biotech companies and validated our solution through 8 user sessions, iterating on feedback. By integrating AI into our flagship analytics application, we enhanced capabilities, sparking increased interest from our 20+ major customers. This new approach positioned the company as a frontrunner in innovative pharmaceutical solutions, enhancing patient safety and setting the stage for continued success in the industry.
50%
Faster detection
95%
User adoption rate
30%
Increase in Efficiency
Measure what matters.